研究室工作进展 Nov. 22nd, 2014
Substituent-Controlled Selective Synthesis of N-Acyl 2-Aminothiazoles
by Intramolecular Zwitterion-Mediated C–N Bond Cleavage
Yang Wang, Fei Zhao, Yue Chi, Wen-Xiong Zhang,* and Zhenfeng Xi
J. Org. Chem. 2014, 79, 11146-11154.
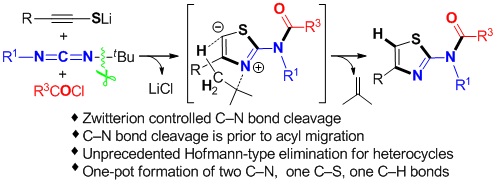
The cleavage of C−N bonds is an interesting and challenging subject in modern organic synthesis. We have achieved the first zwitterion controlled C–N bond cleavage in the MCR reaction among lithium alkynethiolates, bulky carbodiimides, and acid chlorides to construct N-acyl 2-aminothiazoles. This is a simple, highly efficient and general method for the preparation of N-acyl 2-aminothiazoles with a broad range of substituents. The selective synthesis of N-acyl 2-aminothiazoles significantly depends on the steric hindrance of carbodiimides. The result is in striking contrast with our previous convergent reaction giving 5-acyl-2-iminothiazolines via 1,5-acyl migration. It is indeed interesting that the slight change of the substituents on the carbodiimides can completely switch the product structure. Experimental and theoretical results demonstrate the reason why the C–N bond cleavage in the present system is prior to the acyl migration. The intramolecular hydrogen relay via unprecedented Hofmann-type elimination is essential for this totally new zwitterion controlled C–N bond cleavage.
