研究室工作进展, Mar. 19th, 2015
Dianions as Formal Oxidants: Synthesis and Characterization of Aromatic Dilithionickeloles from 1,4-Dilithio-1,3-butadienes and Ni(COD)2
Junnian Wei, Wen-Xiong Zhang, and Zhenfeng Xi*
Angew. Chem. Int. Ed. 2015, DOI: 10.1002/anie.201411009
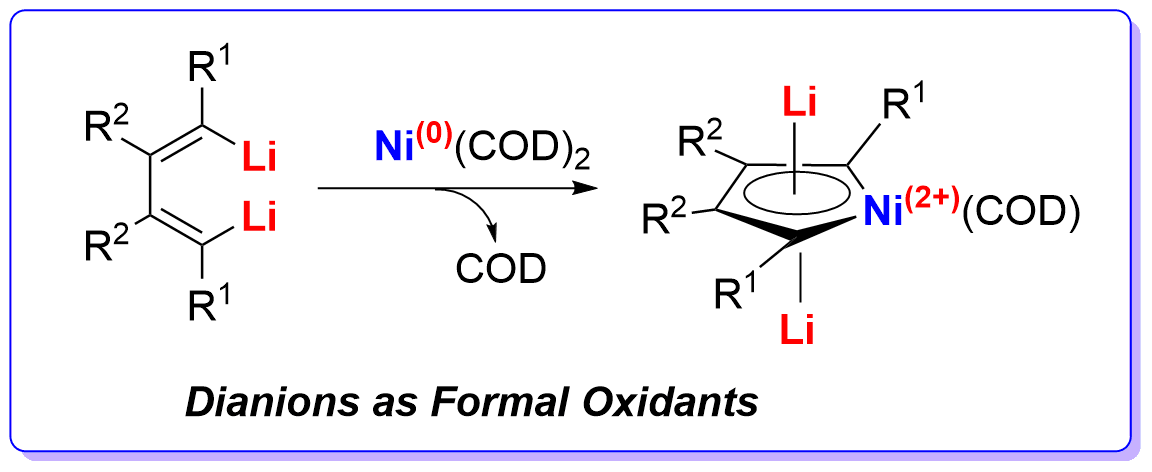
Organolithium compounds can behave as reductants but never as oxidants in redox reactions. Here we report that 1,4-dilithio-1,3-butadienes reacted with Ni(COD)2 to offer dilithionickeloles. Single-crystal X-ray structural analysis revealed a coplanar structure of dilithionickeloles with averaging of bond lengths. XPS data confirmed the oxidation state of Ni in dilithionickeloles was Ni2+. 7Li NMR spectra of dilithionickeloles and theoretical calculations revealed a considerable aromatic character. In this redox reaction, the dilithio dianionic compounds behaved as formal oxidants, oxidizing the Ni0 atom to Ni2+. These results demonstrated that organolithium compounds with π-conjugation could be used as oxidants and could continue to gain extra electrons.
亮点介绍
由于碳负离子的富电本质,在氧化还原反应中碳负离子(如有机锂试剂)可以作为还原剂失去电子,但是从不作为氧化剂得到电子。本工作报道双锂试剂的丁二烯基双碳负离子共轭体系内部轨道的协同效应,与低价金属如零价镍反应时,获得金属镍d轨道的电子从而将零价镍氧化成二价,打破了传统所认为的碳负离子不能够作为氧化剂的认知,为共轭体系碳负离子化学的进一步发展和应用提供了一个全新的思路。该工作再次显示了本组双锂试剂的独特化学性质。
关于该反应产物中镍的氧化态,仅从传统离子型无机化合物的金属氧化态(表观氧化态)概念理解是不合适的。该产物镍杂环构成一个芳香性整体,镍d轨道的电子离域至丁二烯的π*轨道形成6-π芳香体系。传统金属氧化态(表观氧化态)概念适合于描述离子型无机化合物,但是在描述金属有机化合物中金属的氧化态时存在缺陷,需要延伸传统概念或建立新概念。有关金属有机化合物中金属氧化态描述的延伸阅读:M. L. H. Green, J. Organomet. Chem. 1995, 500, 127; A. F. Hill, Organometallics 2006, 25, 4741; G. Parkin, Organometallics 2006, 25, 4744; http://www.columbia.edu/cu/chemistry/groups/parkin/cbc.htm
